We can, and should, do clinical trials in rare diseases
In a recent article, Elie Dolgin tells a story about efforts to develop a drug for the rare cancer subtype that claimed his mother’s life - anaplastic thyroid cancer (ATC). He reviews the recent history of fosbretabulin (Zybrestat), a tubulin-binding drug candidate that disrupts tumor vasculature. In Phase 2/3 clinical trials of fosbretabulin for ATC - a rare indication - fewer patients were recruited than originally planned, and the molecule showed suggestive, but not significant, benefits. The compound is now in a Phase 2 trial for ovarian cancer, a more common indication, and Oxigene, the trial’s sponsor, announced some positive results this spring. Dolgin makes the case that ATC patients are more likely to benefit from off-label use of drugs tested against more common cancers than from clinical trials specifically for ATC, because the disease is so rare and so rapidly fatal as to make drug development infeasible. From this story, Dolgin draws a more general conclusion:
“I give up,” are three words rarely uttered by doctors, scientists, and politicians, and so they continue to try. But for rare, aggressive cancers like ATC, I argue that giving up is exactly what they should do… The enormous amount of money required to bring drugs for ATC and other rare diseases through clinical trials could be used to pursue therapies for more common cancers, with trials that are more likely to succeed, resulting in approved drugs. Once on pharmacy shelves, doctors could prescribe these medicines off-label for patients with no other promising drug options—patients like my mother.
While the story of ATC in particular sounds compelling, after reading Dolgin’s article I worried that readers would take away the message that we shouldn’t do clinical trials in rare disease, or worse yet, that we shouldn’t do research on rare disease at all. That would be a huge mistake.
As Dolgin notes, the ATC trial included 80 participants, while the ovarian cancer trial included 107 participants - only 34% more. ATC has an incidence of less than 0.25 new cases per 100,000 people per year [Davies & Welch 2006†], while ovarian cancer has an incidence estimated at 12.3 new cases per 100,000 per year. Given that ovarian cancer is at least 50 times more common than ATC, why didn’t Oxigene aim to recruit 50 times as many patients for its ovarian cancer trial?
Perhaps it’s because clinical trials are incredibly expensive, and larger patient numbers mean higher costs. Indeed, the pharmaceutical industry is plagued by rising costs and declining approval rates. A recent review argues that clinical trial sizes, which have more than doubled in the past 20 years for certain indications, are one major cause of rising costs [Scannell 2012]. Scannell further makes the point that “Everything else being equal, clinical trial size should be inversely proportional to the square of the effect size. If the effect size halves, the trial has to recruit four times as many patients to have the same statistical power.” Therefore, if we want to more quickly and more cheaply advance more drugs to approval - and patients and drug companies alike should agree that we do - then the key is not to find larger numbers of patients for trials, but to be smarter about which patients we include, so that we can observe larger effect sizes.
And in some cases, being smarter about which patients we include specifically means that we should go after rare indications. A famous (if by now trite) example is that of statins [reviewed in Tobert 2003, Plenge 2013]. The first clinical studies of lovastatin focused on individuals with autosomal dominant familial hypercholesterolemia (FH). With a prevalence of about 1 in 500 [Marais 2004], FH is fairly “common” compared to many rare diseases, yet is far rarer than non-familial hypercholesterolemia. Yet a trial in just 6 individuals with FH [Bilheimer 1983] provided critical evidence regarding the drug’s mechanism, and the first Phase 2 trial, in 101 FH individuals [Havel 1987], provided the earliest evidence of efficacy in humans which helped drive lovastatin to its eventual approval.
Lovastatin can be considered a case of “the rare informs the common”: rare indications that are genetically well-defined and have a severe phenotype offer an opportunity to learn things that will help us treat more common diseases.
Sometimes those opportunities involve learning about the biology of disease, other times they involve learning about the biology of therapeutics. For instance, there is mipomersen, which the FDA approved last year for the treatment of high LDL cholesterol in individuals homozygous for loss-of-function mutations in the LDL receptor gene, LDLR, following a trial in just 51 patients [Raal 2010]. The N was small, but because the trial targeted a highly selected patient population with an extremely well-established biomarker (LDL cholesterol) it was able to observe a large effect (25% reduction, vs. 3% with placebo) that was highly significant (p = .0003). What’s special about mipomersen is that it is an antisense oligonucleotide drug, a class of therapeutic that some observers had wanted to pronounce dead [see commentary in Jiang 2013]. That proof of principle of mipomersen’s success may have been critical to Roche’s $30M deal with Isis Pharmaceuticals last year, paving the way towards a clinical trial of antisense oligonucleotides for Huntington’s disease slated to begin in 2015.
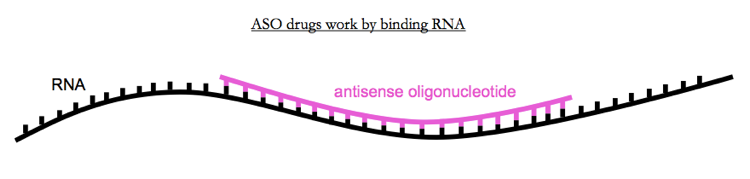
Above: Antisense oligonucleotides (ASOs) are a class of drug that works by binding RNA. An ASO approved last year after a trial in 51 people may help to pave the way for ASOs for more common diseases.
Mipomersen is one example of genomic stratification - defining the target population based on one or more particular genetic mutations. Another success story is ivacaftor for cystic fibrosis, FDA-approved based on a trial in 213 patients with the CFTR G551D mutation. Its approval has since been expanded to several other CFTR mutations, and the royalties on the drug recently sold for $3.3 billion.
Ivacaftor is one example of what Dolgin calls a “direct attack” on a rare disease. Dolgin is negative about such efforts, but they can work. Indeed, Genzyme has made a business of it, developing intravenous enzyme replacement therapies for patients with rare enzymatic deficiencies. One of their drugs, alglucosidase alfa, for patients with loss-of-function mutations in the GAA gene, received approval after a trial in 39 patients.
These are not huge numbers of patients. Far from meaning that we no longer need clinical trials in rare disease, I would argue that genomic stratification makes rare disease trials more feasible than ever before. It just changes the definition of the disease.
In the genomics era, cancers can be defined molecularly - by genetic mutations - rather than by the tissue type of origin. This means that in the future, instead of trials targeting “anaplastic thyroid cancer” as a group, we’ll have so-called “basket trials” combining patients with a variety of “types” of tumors (thyroid, ovarian, and so on) that all share a particular mutation [Willyard 2013]. And saying that we will group patients differently is a very different thing than saying we won’t study rare diseases.
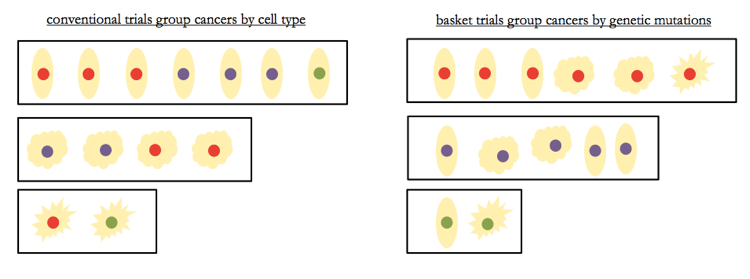
Above: ‘basket trials’ group cancers differently than conventional clinical trials do, but that does not by definition mean larger numbers.
I fully agree with Dolgin that genomics will also be instrumental in helping match patients to existing FDA-approved drugs predicted to work well for them. For instance, Dolgin mentions the example of vemurafenib, which was approved for individuals with melanoma with the BRAF V600E mutation, and was later reported to work well in one individual with ATC with the same mutation [Rosove 2013]. This sort of genetic matchmaking underpins the business model of Foundation Medicine and is likely to prove transformative.
Yet even when two types of tumors do share a genetic mutation, efficacy in off-label use is far from guaranteed. In contrast to the results for ATC, it appears that colorectal tumors with the BRAF V600E mutation do not respond to vemurafenib [Prahallad 2012]. Oncologist and cancer genomics researcher Nikhil Wagle points out that “If one assumed that vemurafenib would be as effective in all other BRAF mutant cancers as it is in melanoma, a lot of patients might have received an ineffective therapy. Moreover, since the off-label use would be occurring outside the context of a study, we wouldn’t have been able to learn that it wasn’t effective until many, many patients had failed therapy.” Instead, he says, even after a drug is approved, we still need to be rigorous about assessing its efficacy in other indications. This could include clinical trials of new indications for approved drugs, basket trials of approved drugs, and the development of an “off-label registry” as recently proposed by Richard Schilsky [Schilsky 2014]. Indeed, Dolgin mentions a second ATC patient who was successfully treated with the FDA-approved drug everolimus, as an example of a success story in off-label use [Wagle 2014b]. But that wasn’t an off-label use: that patient was enrolled a Phase 2 clinical trial of everolimus in thyroid cancer. The Dana Farber Cancer Institute has now launched a basket trial of everolimus for individuals with TSC1 or TSC2 mutations, inspired by that individual and two other patients who responded exceptionally well to everolimus - “extraordinary responders” - both of whom were likewise identified in conventional clinical trials [Iyer 2012, Wagle 2014a].
The possibility of off-label use, then, does not free us from the need to learn from clinical trials, and to design new trials based on genomic insights. Moreover, to enable off-label use, we still need to get new drugs approved in the first place, which is where genomics can help us to carefully design clinical trials targeting tightly defined patient populations. Indeed, vemurafenib is just one of several drugs approved for tumors with a specific genetic mutation. Crizotinib was designed specifically for non-small cell lung cancers with translocations in the ALK gene, and its efficacy in this specific patient group was so profound that it received FDA approval following positive results from only its Phase 1 and Phase 2 trials, on a total of just 255 patients [reviewed in Ou 2011]. Its efficacy has since been confirmed in Phase 3 in 347 individuals [Shaw 2013]. Note here that genomic stratification doesn’t necessarily mean we need more patients in trials, it means we need the right patients. Gefitinib had its approval restricted by FDA after a study without genomic stratification in 1,692 lung cancer patients showed no benefit [Thatcher 2005], even as much smaller studies demonstrated that it worked brilliantly against tumors with EGFR mutations [Paez 2004, Lynch 2004].
By this point I’ve touched on a lot of different drugs. Sometimes the rare has informed the common, sometimes drugs for more common diseases are used off-label for rare indications, and sometimes we can succeed at treating the rare for the sake of the rare. I mention all of these examples not to illustrate a general trend, but rather, to illustrate the lack of one. There is no simple rule for what makes a good drug, or an approvable drug.
As a statistical geneticist, I am a huge believer in power calculations, and I am not advocating that we proceed with clinical trials that have no statistical power. But power depends not only on sample size, but also on effect size, and on variance in patient outcomes. As mentioned above, Scannell has lamented that half the effect size means you need four times the sample size. A happy correlate of this observation above is that if you double the effect size, you only need one quarter the number of patients. Today we have unprecedented tools available to help us identify the specific patient populations for whom drugs are most likely to be highly effective. We can, and should, use those tools to find new treatments for rare diseases.
