Organic chemistry 20: Alkenes - oxymercuration, hydroboration
These are my notes from lecture 20 of Harvard’s Chemistry 20: Organic Chemistry course, delivered by Dr. Ryan Spoering on March 27, 2015.
Alkene hydration with mercury
Alkene hydration refers to converting alkenes to alcohols. The simplest way to do this is with a weak nucleophile, which will give essentially the same results as SN1. For instance, these two reactions can be completed simply with acid and water, in each case giving the Markonikov product.

The trouble is that this is not scalable to more complex molecules. The more functional groups you have, the more likely that something else will also react with the H2SO4, and the more likely that your carbocation intermediate will give rise to some sort of rearrangement.
Using mercuric acid instead gives you far greater specificity for alkenes. Here, we use mercuric acetate in water to achieve the same reaction as at top, but in a milder fashion, such that no rearrangements will occur. The downside is this that this requires mercury, which puddles out at the bottom of the reaction in the second step and has to be handled carefully.
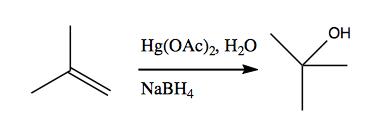
Here is the mechanism:
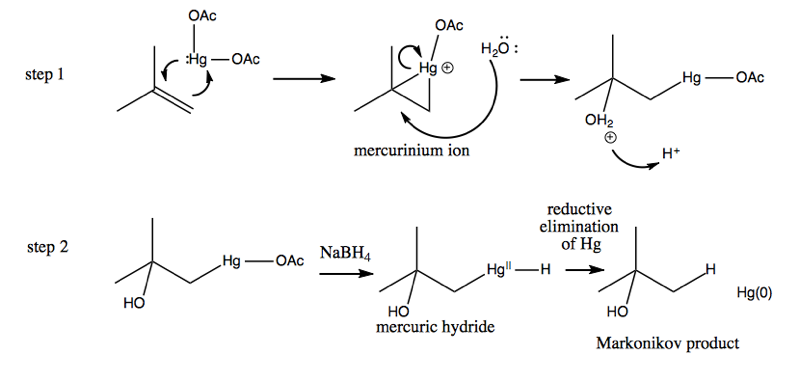
The “reductive elimination of Hg” step is left as a black box for now; it will be discussed next week.
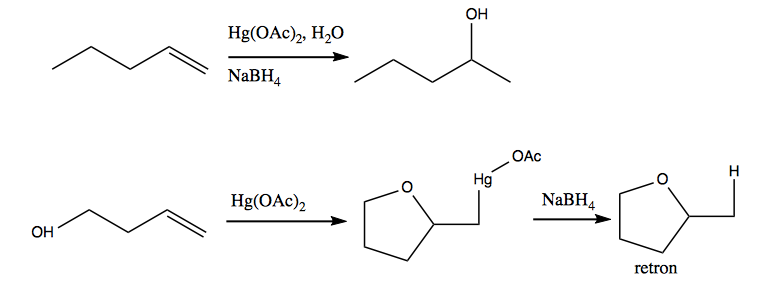
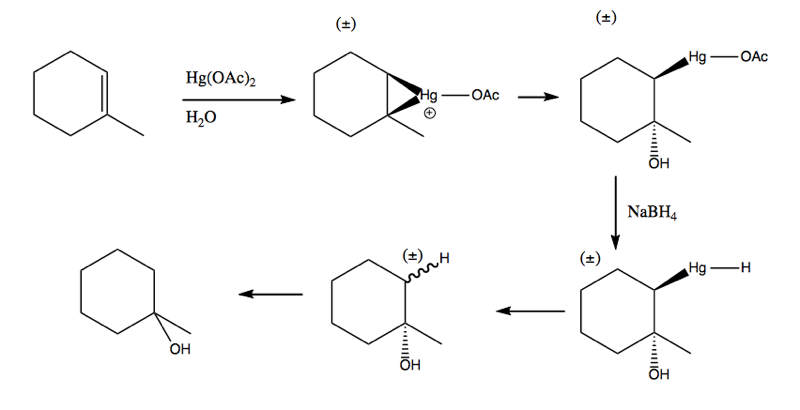
Here are two more examples of what mercury acetate can do:
Here is an example where it actually gives rise to a racemic intermediate. However, the stereogenicity of that particular carbon is rarely important anyway.
Hydroboration
The study of hydroboration is attributed to H.C. Brown. He figured out that diborane has the structure at below left. Using tetrahydrofuran (THF) you can disrupt the hydrogen bonding and get a BH3·THF complex (right), which is highly reactive with alkenes.
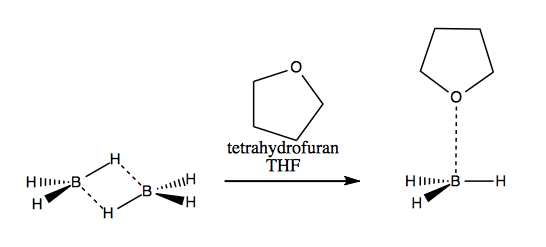
By reacting this complex with an alkene you can get organoborane:
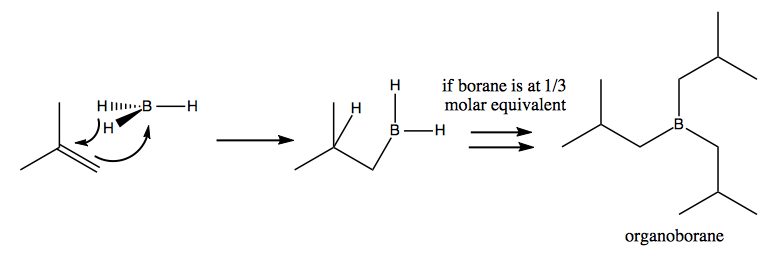
Through oxidation with hydrogen peroxide you can then get an organoborate ester. By hydrolysis you then get three copies of the alcohol, plus B(OH)3:

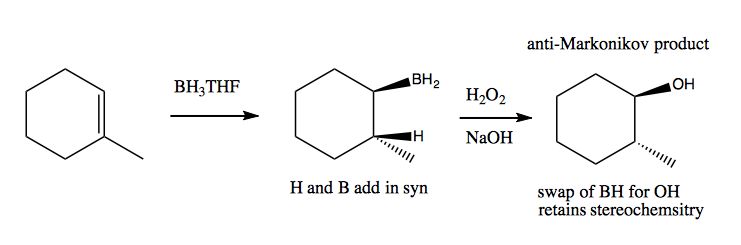
BH3·THF can retain stereoselectivity and yield an anti-Markonikov product:
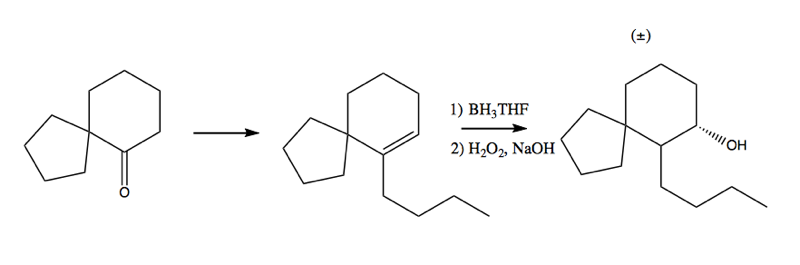
Hydroboration is critical for the synthesis of this interesting molecule:

It takes several steps but the key insight that enabled it synthesis was the use of BH3THF to add the -OH group in the second step:
There are all sorts of organoborane derivatives that you can use for different reactions. For instance, 9-BBN (below) is useful for achieving selectivity when converting terminal alkenes to alcohols. The steric bulk of 9-BBN forces the B to be added at the ultimate rather than penultimate carbon.

