cpd-B as a prion therapeutic
 Read with caution! This post was written during early stages of trying to understand a complex scientific problem, and we didn't get everything right. The original author no longer endorses the content of this post. It is being left online for historical reasons, but read at your own risk. |
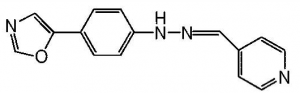
After hearing a few times about a mysterious “compound B” which has been tested as a potential prion therapeutic, I finally got around to digging up the citation: Kawasaki..Doh-Ura 2007 ”Orally Administered Amyloidophilic Compound Is Effective in Prolonging the Incubation Periods of Animals Cerebrally Infected with Prion Diseases in a Prion Strain-Dependent Manner” . The researchers tested a battery of different styrylbenzoazole derivatives in vitro and in mice and found one, the so-called “cpd-B”, which was quite effective: it doubled or even tripled the incubation time in mice, depending upon the dosage used. Here’s what cpd-B looks like:
In order from left to right, that’s the azole, the benzene, and the styrene. Styrylbenzoazole.
Doh-Ura had gotten interested in this group of compounds after Okamura 2004 showed that they can bind to amyloids in the brain to enable imaging of aggregates in Alzheimer’s and Kawasaki 2006 showed they would also bind to prion aggregates. This all seems to be part of a larger backstory I’m just discovering: in the 1990s people using Congo red, a common laboratory stain, to image prions in vitro noticed that Congo red actually inhibited aggregate formation. Congo red is both toxic and BBB-impermeable, though that didn’t stop people from considering it as a therapeutic [Caughey 1992]. Interest eventually shifted towards styrylbenzoazoles (which are not chemically similar to Congo red) instead, in part due to the efforts of Doh-Ura’s lab.
This study used four different animal models: Tga20 (mice overexpressing mouse PrP), Tga7, (mice overexpressing hamster PrP), ICR mice (by which I think they mean outbred mice e.g. this, this, rather than genomic imprint mice), and Syrian hamsters. And it used four different prion strains: RML1, 22L, Fukuoka-1 (from human GSS brains), and 263K (hamster scrapie). Six of the possible 4×4=16 possible combinations of animal and prion were studied. In each case the animals were infected by intracerebral brain homogenate injection.
Of the compounds studied, apparently the others had no effect, as most of the paper is devoted to discussing cpd-B. cpd-B was highly effective for some prion strains and not at all effective for others [Fig 2]. Specifically, cpd-B worked extremely well for RML prions: it doubled or even tripled incubation time in Tga20 mice, behaving in a dose-dependent manner, and it appeared to do the same in ICR mice. (ICR mice are outbred, therefore healthier by nature, and also don’t overexpress PrP, therefore less vulnerable to prion infection than Tga20 mice. So the researchers didn’t let the study go on long enough to see what would eventually happen with all of the treatment group ICR mice, but the survival curves look like incubation time was about doubled.) cpd-B also worked reasonably well for Fukuoka-1 prions, with more like a 30% increase in incubation time. It worked minimally for 22L and not at all for 263K prions.
The authors also investigated the pharmacological properties of cpd-B by measuring its concentration in the animals’ brains. As for toxicity, some weight loss was observed only in animals treated with the very highest dose of cpd-B; no other noticeable side effects were observed, even in uninfected mice treated with cpd-B for a year. As for permeability, cpd-B was found to be blood brain barrier-permeable: when it was administered orally, “a considerable amount of cpd-B was detected in the brains of all experimental animals”, and when it was injected into the tail vein, “the ratio of the cpd-B concentration in the brain to that in the blood plasma is 2.7:1, indicating that cpd-B is equal to the best brain-entering amyloidophilic chemicals previously identified”. However, it also appears to be cleared by the body very rapidly: “both the brain level and the blood plasma level of cpd-B at 30 min after the intravenous injection were below the measurable level of 50 pM, which indicates that cpd-B is very rapidly washed out from the brain and blood.” The doses of cpd-B used were quite large: 150, 225, 300 and 500 mg/kg body weight/day– using the table from Reagan-Shaw 2008, that works out to a range of 850 – 2800 mg/day for a 70 kg person. In the conclusion the authors note that the rapid clearance of cpd-B from the body accounts for the need for such large doses, and that there is a need to pharmacologically improve this compound for better retention in the brain.
This study had a number of other facets as well: in vitro experiments on mouse neuroblastoma cells (N2a cells) showed that cpd-B inhibited PrPres formation of RML prions but had little effect for the other strains, and another in vivo experiment showed that materials from infected mice treated with cpd-B were less infectious than materials from untreated mice.
All this seems fairly promising. It’s hard to extrapolate directly into therapeutic potential for the human prion diseases because of the strain-dependent nature of the compound’s effect. But this does seem to merit further testing, and in particular, I’m curious if chemically similar compounds could be derived that would each have affinity for a different prion strain. This study was published five years ago, and a Google search did not reveal any more recent hits for “cpd-B” or “styrylbenzoazole”. If anyone knows what the current state of research is in this area, let me know!