Cell Biology 11: Apoptosis & Necrosis
These are notes from lecture 11 of Harvard Extension’s Cell Biology course.
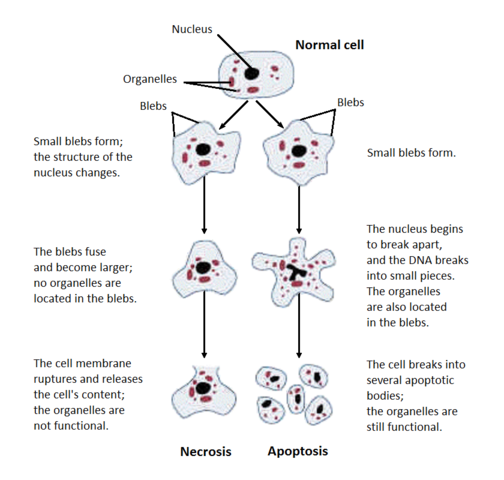
This lecture will cover two different ways cells can die: apoptosis (programmed cell death) and necrosis (unplanned cell death). It is easy to tell these two apart morphologically under the microscope, as shown in this Wikimedia Commons image:
necrosis
Necrosis is when cells die accidentally due to, say, trauma (ex. a poisonous spider bite), or lack of nutrients (ex. lack of blood supply). Necrosis begins with cell swelling, the chromatin gets digested, the plasma and organelle membranes are disrupted, the ER vacuolizes, the organelles break down completely and finally the cell lyses, spewing its intracellular content and eliciting an immune response (inflammation).
apoptosis
Apoptosis can constitute cell suicide or cell murder. Cells will commit suicide when they lack any incoming survival signal in the form of trophic factors, or when they detect extensive DNA damage in their own nucleus. Cells will murder other cells to clear out unneeded cells or to eliminate potentially self-attacking immune cells.
Either of these processes constitutes programmed cell death. During embryonic development, people have webbed hands and feet and tails; the cells that constitute those parts later apoptize. Apoptosis also goes on constantly in many tissues including the intestines.
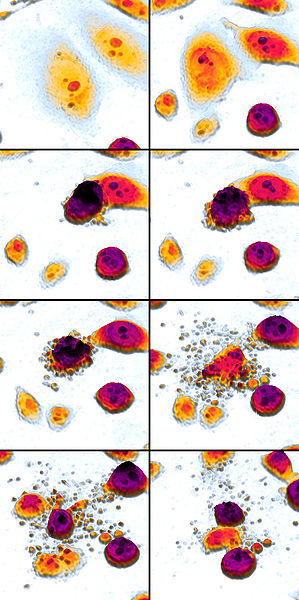
Here’s a stunning Wikimedia Commons image of apoptosis (read left to right, top to bottom) thanks to Egelberg:
Major steps of apoptosis:
- Cell shrinks
- Cell fragments
- Cytoskeleton collapses
- Nuclear envelope disassembles
- Cells release apoptotic bodies
Notably absent from this list is ‘send out a signal.’ Apoptotic cells do not send out any signal, with one exception: they release apoptotic bodies and ‘engulfment proteins’ to induce other cells (‘phagocytic’ cells) to engulf the apoptotic bodies and and break them down in their lysosomes, but this is not much of an immune response.
Proteins important in apoptosis:
- ‘killer proteins’: the caspases (discussed in detail below).
- ‘destruction proteins’ that digest DNA, fragment the cell and break down the cytoskeleton
- ‘engulfment proteins’ that elicit and promote phagocytosis by other cells
C. elegans has been the major model organism for understanding apoptosis, both by forward and reverse genetics. Forward genetics is observing a phenotype and then determining which gene gives rise to it; reverse genetics is introducing a mutation into a known gene in order to see what phenotype results.
The key pathway in C. elegans apoptosis is shown in this Google Drawing I created:
Here’s an explanation of how each of these proteins does its job, from bottom up:
are:
- CED-3 pulls the trigger, activating apoptotic proteins that destroy the cell. (In the mammalian equivalent, CED-3 is Caspase 9, which cleaves-thereby-activating Caspase 3, which in turn destroys the cell.)
- CED-4 activates CED-3.
- CED-9 binds to CED-4, preventing its activation
- EGL-1 is transcriptionally activated in response to death signals and catalyzes the release of CED-4 from CED-9.
Note that there is no robustness in this system – it is single points of failure all the way through. If CED-3 is knocked out, no apoptosis can occur. If CED-4 is knocked out, no apoptosis can occur. If CED-9 is knocked out, every cell in the worm will apoptose. If EGL-1 is knocked out, no apoptosis can occur. Note that the order the arrows point in the above diagram reflects the flow of information in the system. For instance, if EGL-1 and CED-9 are both knocked out, it’s the same as if CED-9 alone was knocked out: every cell will apoptose.
In mammals, apoptosis is governed chiefly by caspases (cysteine-aspartic proteases). The entire caspase pathway is post-translationally regulated: the caspases are always present in inactive form (called procaspases, containing a prodomain, which contains a caspase recruitment domain (CARD)) and can be activated by cleavage. This allows a very quick response if cell suicide is needed. In order for apoptosis to occur, the initiator caspases must be cleaved and dimerize. Thus activated, they must then cleave the effector caspases (aka pro-caspases), triggering a ‘caspase cascade’. This amplifies the number of activated caspases in the cell. The effector caspase have many targets including the nuclear lamina and cytoskeleton.
There are both pro-survival and pro-apoptotic caspases, and they share many common domains. Pro-survival caspases have BH1, 2, 3 and 4; pro-apoptosis caspases have either BH1, 2 and 3 or just BH3.
Inhibitor of apoptosis proteins (IAPs) restrain both the initiator and effector caspases. They each have a zinc binding domain that binds directly to caspases, inhibiting their activity.
However, there are also mitochondrial proteins called SMAC and DIABLO which inhibit the inhibitors. Upon mitochondrial injury they are released and will bind IAPs, freeing the caspases to go cause apoptosis. Another collection of mitochondrial proteins called Htra2/Omi, apoptosis-inducing factor (AIF) and endonuclease G can also be released and will cleave IAPs. AIF also causes chromosome condensation and DNA fragmentation independent of caspases.
Indeed, the mitochondria are central regulators of apoptosis. Outer mitochondrial membrane proteins Bcl-2, the BH3-only proteins and Bax are involved: Bax can form a pore in the membrane to allow cytochrome c, normally located in the intermembrane space, out into the cytosol. Bax monomers move from the cytoplasm to the outer mitochondrial membrane, where they oligomerize and permit the influx of ions through the membrane. This has also been shown in in vitro experiments where you can show that vesicles made of outer mitochondrial memrbanes are permeabilized in the presence of Bax. It is not currently known why this influx of ions leads to cytochrome c release.
Bcl-2 prevents release of cytochrome c, thus blocking apoptosis. Bcl-2 was the first mammalian apoptosis gene to be cloned. In some lymphomas, it gets translocated to a position under a stronger promoter, causing overexpression that prevents the cancer cell from apoptosing. See also bad & bid.
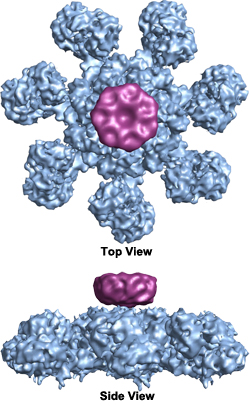
Once cytochrome c is released, it binds to Apaf-1 (apoptotic protease activating factor), causing the latter to hydrolyze the ATP to which it is usually bound, thus causing a conformational change that activates Apaf-1 and triggers the caspase cascade. Apaf-1 forms a disc-shaped heptamer called the ‘wheel of death’ or apoptosome which activates caspases (Wikimedia Commons image by Org1012):
When a trophic factor is present, the receptor activates PI3K, which activates PKB/Akt, which phosphorylates Bad. p-Bad is then retained in the cytosol by 14-3-3, preventing p-Bad from inhibiting Bcl-2. Thus apoptosis is prevented.
Trophic factors are an example of a cell extrinsic signal that promotes survival. There are also extrinsic signals that promote death (this is cell murder). Tumor necrosis factor (TNF-alpha) is released by macrophages to trigger cell death by binding to ‘death receptors’. Death receptors have a single transmembrane domain. They must trimerize in order to activate FADD (Fas-associated death domain). These serve as adapters for caspase-8 and -10 and form a death-inducing signaling complex (DISC) which can initiate the caspase cascade. Though this whole process originates independent of mitochondria, it can also activate (?) t-Bid, leading to a mitochondrial apoptosis signal as well.
Cells can become murder-resistant by expressing decoy receptors which have only the ‘death ligand’ binding domain and no active cytosolic domain. This occurs sometimes normally in animal cells but is also a trick that some viruses use – they encode decoy receptor proteins to keep their host cells safe from immune attack.
TNF-alpha usually promotes death, but can also promote survival in certain cell types by activating NF-κB. Sometimes cells use decoy receptors to promote an inflammatory response instead of death.
p53 is a key regulator of DNA damage response and can promote DNA repair, apoptosis or cell cycle arrest. It does this by binding to promoters of target genes. It is still not clear what determines when p53 will induce cell cycle arrest versus apoptosis.
experimental methods
Apoptotic cells exhibit a particular chemical signature. One of these is that an endonuclease cleaves DNA into fragments in the linker regions between nucleosomes and the resulting fragments form a ladder when run on a gel. Another is TUNEL (Terminal deoxynucleotide transferase dUTP Nick End Labeling) staining. This involves adding a Tdt enzyme and a BrdU which Tdt will add to the ends of cleaved DNA. After giving it a chance to do this you wash away excess BrdU and then use an antibody against BrdU. Yet another method is that phosphatidylserine (PS) is normally located in the cytosolic leaflet of the plasma membrane; during apoptosis, it flips to the exoplasmic leaflet, where it serves as a signal to request other cells to phagocytose the dying cell. A fluorescently labeled annexin V protein can label PS on the outside of apoptotic cells.
Double-stranded DNA cannot get through the plasma membrane of intact cells – and that means healthy cells and apoptotic cells. If it does get out, that is a sign of necrosis. So you can stain with annexin V for exoplasmic PS and with 7-AAD for dsDNA; apoptotic cells are those which are positive for annexin V but negative for 7-AAD.
concluding video
In sum, here is a disturbing video about apoptosis:
relevance to PrP
In general, in nature, cells either die by apoptosis, necrosis or by autophagy (meaning, in this case, getting engulfed whole by other cells). There aren’t really any other ways to go. One of the many mysterious things about prion diseases is how neurons die in the prion-infected brain – they do not obviously appear to follow any of these paths. Here’s a quote from an excellent recent paper on toxic mechanisms of prion disease [Moreno 2012]:
Caspase 12 cleavage occurred at 10wpi, following rising CHOP expression… coincident with onset of neuronal loss… however the exact effector mechanism of neuronal death is unclear: we found neither apoptosis, nor autophagy, nor necrosis on examination of hippocampal slices… and neither Bax deletion, nor Bcl-2 overexpression, nor caspase 12 deficiency are neuroprotective in prion disease.
In addition to her own evidence, Moreno cites the study of prion infection in mouse models with Bax (a pro-apoptotic protein) deleted or Bcl-2 (an anti-apoptotic protein) overexpressed – two different ways of blocking apoptosis. Neither of these mouse models had any delay or amelioration of prion disease [Steele 2007a]. Another apoptotic protein, Caspase-12, undergoes proteolytic processing during prion infection, but deletion of Caspase-12 also did not change the course of prion disease [Steele 2007b].