Organic chemistry 09: Introduction to substitution and elimination
These are my notes from lecture 9 of Harvard’s Chemistry 20: Organic Chemistry course, delivered by Dr. Ryan Spoering on February 20, 2015.
Substitution
We’ve already seen one example of a substitution reaction:
CH
Here, Br- is the leaving group.
A different type of reaction involving a similar set of substrates is a β-elimination reaction:
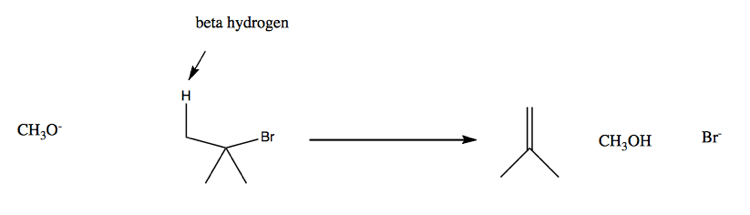
A group’s ability to be a leaving group is somewhat correlated with the acidity of its conjugate acid. Strength of acidity, or pKa, is basically a measure of ability to leave hydrogen, whereas a leaving group has to leave carbon, so it is a helpful but imperfect predictor:
| X | pKa of HX | leaving group quality |
|---|---|---|
| I- | -9 | good |
| Br- | -9 | good |
| Cl- | -7 | good |
| RSO3- | -2 | good |
| ROH, H2O | -2 | good |
| F- | 3 | ok |
| carboxylates | 5 | ok |
| CN- | 9 | ok |
| -OH | 16 | bad |
| -OR | 16 | bad |
Substitution and elimination can each proceed via one of two mechanisms: unimolecular (dissociative) or bimolecular (concerted).
SN1: Unimolecular (dissociative) substitution
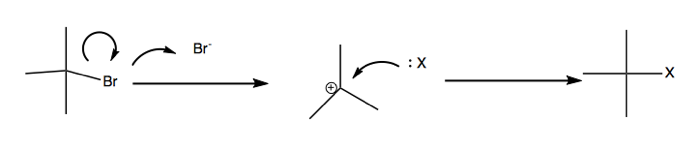
Above, the departure of bromide is the rate-limiting part. Once you have formed a carbocation it is highly reactive and the second step will proceed rapidly.
SN2: Bimolecular (concerted) substitution
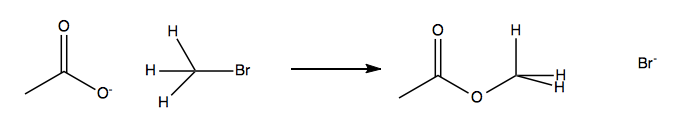
Here, the leaving does not happen on its own - there has to be a nucleophilic attack to release it.
E1: Unimolecular beta elimination
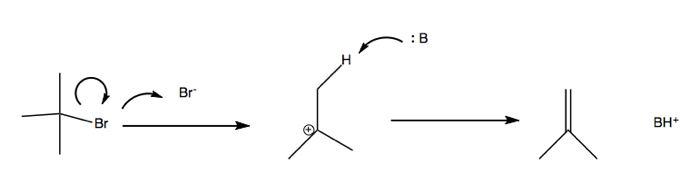
E2: Bimolecular beta elimination
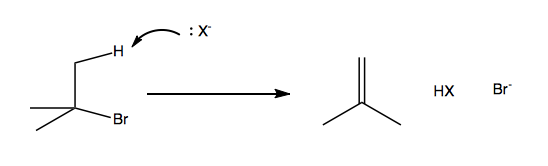
Coming up, there will be one lecture entirely devoted to each of these mechanisms. Alkyl halides are very common and useful reagents for these reactions, so we’ll focus on them.
We’ll consider CH3Br, properly bromomethane but we’ll call it methyl bromide. We’ll also consider three isoforms of butyl bromide. These have their own nomenclature because they have very different reactivities.
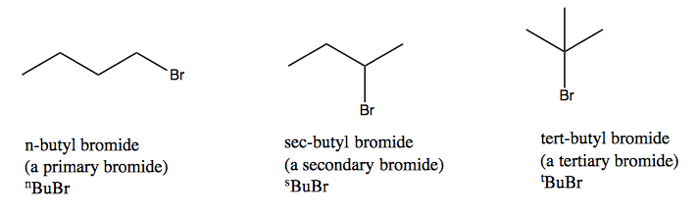
When a halide is bonded directly to a C that has a double bond, it is called a “vinylic halide”. If it is bonded directly to an aromatic ring it is an “aryl halide”. Both of these are non-reactive - because of the adjacent bond, they will not act like alkyl halides in the S1, S2, E1 and E2 reactions diagrammed above.
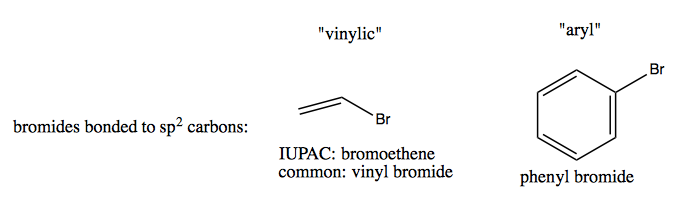
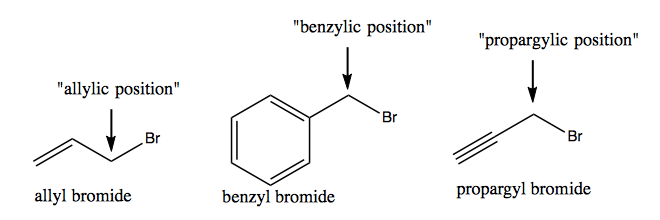
Positions of carbons in alkenes and alkynes get many special names, none of which are recognized in IUPAC nomenclature but all of which are very commonly used. Here are a few examples:
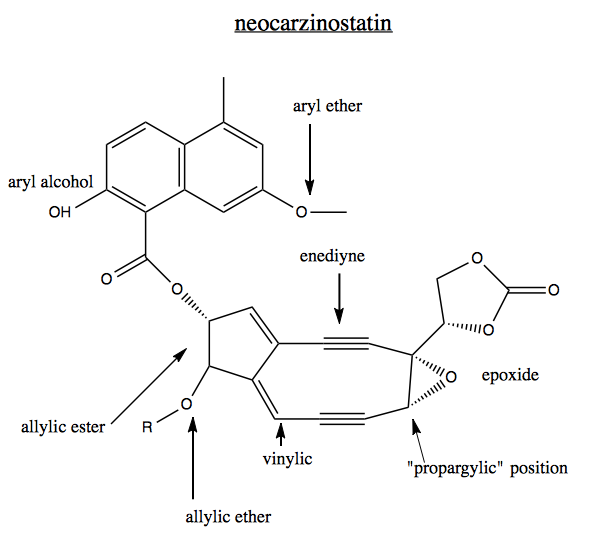
The following natural compound has enediyne ring with two triple bonds. This is referred to as a “warhead” because it allows the molecule to intercalate DNA and break it. This compound is an antibiotic. It is a great example of just how many names there are for positions and functional groups.
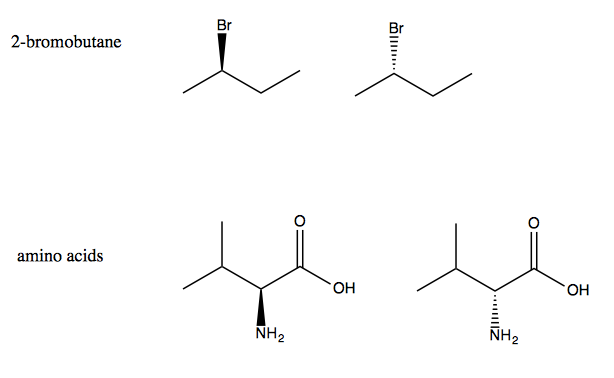
Moving forward we’ll need to consider the stereochemistry of compounds. 2-bromobutane comes in two forms, analogous to L and D amino acids.