Organic chemistry 19: Alkenes - epoxidation, dihydroxylation, cyclopropanation
These are my notes from lecture 19 of Harvard’s Chemistry 20: Organic Chemistry course, delivered by Dr. Ryan Spoering on March 25, 2015.
Epoxidation
The reagent mCPBA adds an oxygen to one face of an alkene:
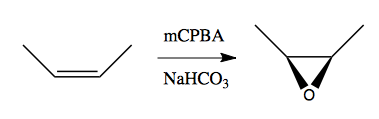
This is stereospecific:

Except of course in cases where the faces are homotopic:
mCPBA is explosive, so it’s usually shipped as a 70% mixture with water and carboxylic acid.
You’ll recall from lecture 18 that bromination with Br2 involves two simultaneous molecular orbital exchanges:
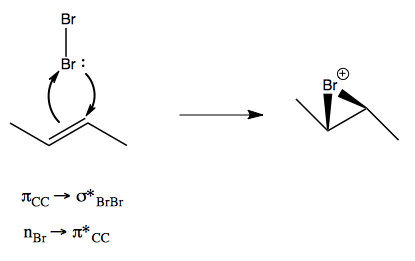
Well, the mechanism of mCPBA is highly analogous:
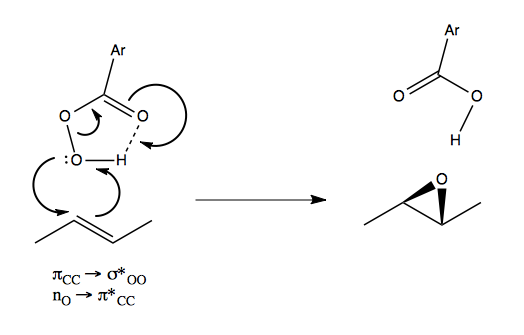
Dihydroxylation
Osmium metal is the densest naturally occurring substance. It oxidizes in the presence of air to form osmium (VIII) tetroxide (OsO4), which is highly toxic. It is a deep black solid that produces black fumes that can stain many things black, including the whites of your eyes. Its usefulness, however, is so profound that people tolerate dealing with it depsite the danger. It is versatile and robust to the presence of many other functional groups - here’s what it does:
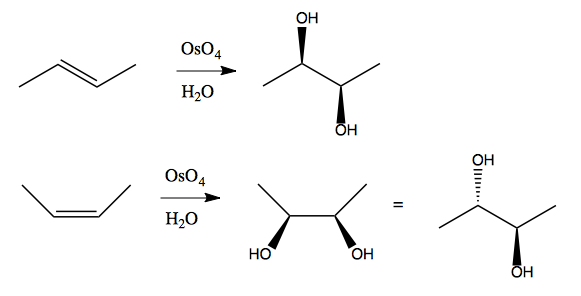
And here is its mechanism:
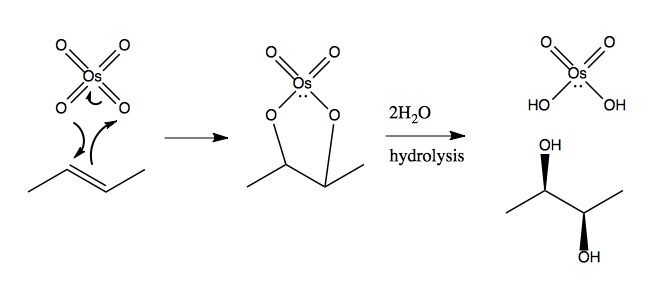
Aside: oxidation and reduction
Oxidation is the loss of electrons, reduction is the gain of electrons. Consider the progressive oxidation of this primary alcohol:
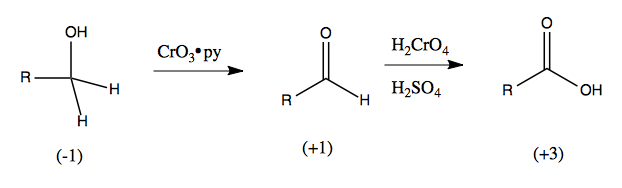
Because oxygen is more electronegative than carbon, it owns the electrons in the C-O bond. Because carbon is more electronegative than hydrogen, it owns the electrons in the C-H bonds. Carbon therefore has one negatively charged group bound to it (the -OH) and two positively charged groups (the -H). Therefore the carbon’s oxidation state is 1(-1) + 2(+1) = -1. If we then oxidize it with Collins reagent, it now has an oxidation state of +1. If we oxidize it further with Jones reagent, we have three negatively charged groups, for a +3 oxidation state. As we do this are just adding more and more electronegative elements to the carbon, thus removing electrons from the carbon. Organic chemists do not often count oxidation states.
Thinking back to either the epoxidation reaction at top or the OsO4 reaction above, the two electrons in the π bond of the alkene end up in a C-O bond instead. This represents a loss of 2 e- from carbon, for a +2 increase in oxidation state. For contrast, consider the hydrogenation of an alkene with Pd/C. Here, carbon gains 2 e- from the H2 that arrive, for a -2 change in oxidation state.
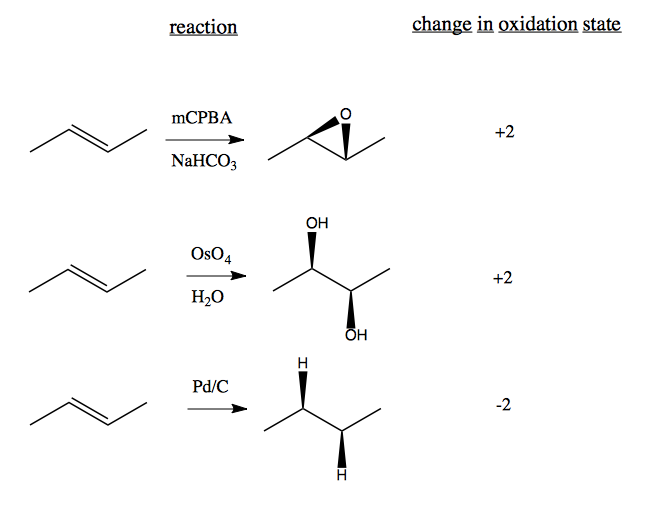
Cyclopropanation
Chloroform (CHCl3) can be used to add cyclopropane to things:
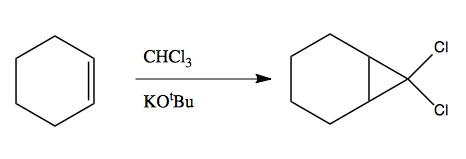
This is because chloroform has a pKa of ~15. The oxygen in KOtBu will attack the H in chloroform, and then one Cl will leave with the extra electrons, giving you a neutral carbon owning 6 e-. This compound is called carbene.
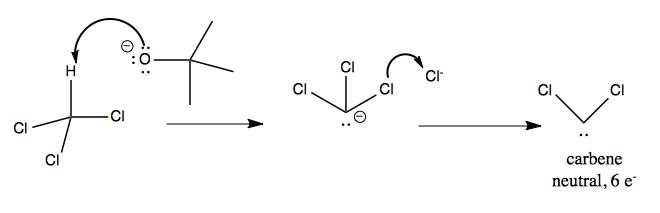
The lone pair on carbene is sp2 hybridized with the two C-Cl bonds, while there is an empty p orbital. Carbene will attack one face of a double bond in an alkene, yielding cyclopropane.
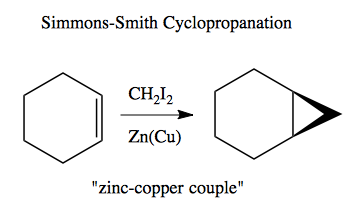
Then there is also Simmons-Smith Cyclopropanation: