Cell Biology 09: Signal Transduction
These are notes from lecture 9 of Harvard Extension’s Cell Biology course.
overview of signaling
Signaling is how cells and organisms get inputs from their environment, and how cells in multicellular organisms communicate with each other. In terms of differentiation, signaling in embryonic development allows establishment separate developmental lineages such as endoderm vs. mesoderm vs. ectoderm and in adult organisms for proper differentiation of stem cells. In terms of environmental response, signaling includes everything from migration of cells in response to growth factors up to fight or flight responses to environmental threats to the organism.
Signaling molecules are synthesized in signaling cells and then released to affect other receiving cells by binding to a target receptor. In some cases that receptor affects a second messenger inside the recipient cell.
Signal transduction involves the following steps:
- Synthesis of signaling molecule by signaling cell (e.g. hormones by pituitary gland)
- Release of signaling molecule (e.g. into blood or extracellular matrix)
- Transport to receiving cell (e.g. in blood)
- Binding to receptor
- Initiation of intracellular signal transduction
- Resultant changes to cellular functions functions (e.g. activating enzymes would be a fast response, changing gene expression would be a slower response)
- Feedback regulation: removal of signaling molecule or disabling of receptor (e.g. via endocytosis)
Most signaling molecules are too large and/or hydrophobic to get through the cell membrane, hence the need for protein receptors on the receiving cell’s membrane. Receptors are usually integral membrane proteins and the binding site is usually located in the strictly extracellular portion but is sometimes in the membrane spanning domain. Binding of the signaling molecules (aka first messengers) to these cell surface receptors leads to an increase or decrease in concentration of intracellular signaling molecules (second messengers) which bind other proteins to modify their activity.
Rapid responses to environmental signals usually go through the nervous system and travel via hormones (insulin, epinephrine, dopamine for instance) synthesized in places such as the pancreas, pituitary glands, hypothalamus or other neurons. Signaling molecules are synthesized in the cytosol, trafficked through the secretory pathway and then held inside the cell until a signal indicates to exocytose them. Such signals usually lead to ‘short term responses’ unless of course the cell is exposed to the signal for an extended period of time.
Here are the different types of signaling:
- Endocrine: molecules synthesized and released by signaling cells travel through blood and act at a distance (e.g. hormones)
- Autocrine: when cells respond to substances that they release themselves (e.g. interleukin-1)
- Paracrine: cells respond to substances released by nearby cells (e.g. neurotransmitters, growth factors). These sometimes form a concentration gradient resulting in a gradient of the degree of cellular response.
- Intracrine: intracellular signaling, not covered in this lecture
Sometimes membrane-bound signals on one cell bind to receptors on adjacent cells to trigger differentiation. Here the membrane proteins themselves are the ligands. Sometimes the membrane proteins are cleaved and become solubilized and then may even act at a distance.
experimental techniques
One experimental technique for finding a protein that acts as a receptor for a particular signaling molecule is affinity chromatography:
- Conjugate the ligand (signaling molecule) to a bead
- Expose beads to cellular extract
- Wash away anything that didn’t bind to a bead
- Now add a lot more signaling molecule. The receptor’s affinity for the ligand is not absolute, they associate and dissociate frequently, so some receptor will dissociate from the bead-bound ligands and associate with the free ligand.
- Collect and purify the ligand/receptor complexes
- Determine identity of receptor peptide (e.g. through mass spec?)
- Identify the gene coding for that peptide
- Express that gene on a cell type that you know does not normally express it, and test if that causes that cell type to bind the ligand.
Similarly, molecular cloning can be used. Simply take a cell type that doesn’t bind the ligand and screen a library of cDNAs to find those which cause the cell to bind the ligand.
Alternately, if you know the impact of cell A’s signal on cell B, you can use mutagenesis screens. For instance if you know that cell A causes cell B to become an R7 neuron instead of a cone cell, you can screen for mutants that become a cone cell. Those mutants might have the receptor for that particular differentiation signal disabled. But other mutations in the relevant pathway can create false positives, so this is a tricky technique.
One way to test the function of G protein coupled receptors (introduced below) is via Fluorescence Resonance Energy Transfer (FRET) which lets you get a readout of the proximity of two different proteins.
G protein coupled receptors
The largest class of receptors is G protein coupled receptors (GPCRs). There are ~900 of them. Activation of these receptors alters gene expression and can lead to differentiation. The mechanism is often that the ligand binding has GEF activity: it causes a conformational change in the GPCR that exchanges GDP for GTP. A separate GAP will later act as an ‘inactivator’, causing the G protein to hydrolize its GTP, leaving it bound to GDP and thus in an inactive state.
Epinephrine is a hormone that binds to a GPCR. In the liver it can cause an increase in cAMP levels leading to glycogen breakdown and glucose release; in muscle it increases intracellular Ca2+ and promotes muscle contraction.
All GPCRs are 7-pass transmembrane proteins with the N terminus extracellular and C terminus cytoplasmic, thus having 4 extracellular and 4 cytosolic domains (called E1-4, C1-4 respectively). They associate with trimeric G proteins (see below), usually via their C2, C3 and C4 domains. The exoplasmic surface consists mainly of hydrophobic amino acids. The cytosolic amino acids vary.
Broadly, there are two classes of G proteins involved in signaling:
- Monomeric G proteins such as Ras are just a single G protein which acts as an on/off switch according to its GTP binding (promoted by GEFs) and GTP hydrolysis (promoted by GAPs). Some of these are involved in differentiation, apoptosis, etc. and so are frequently mutated in cancers.
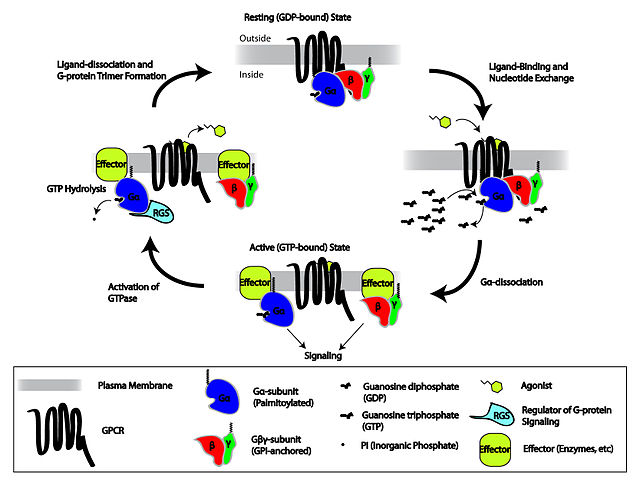
- Trimeric G proteins are complexes of three proteins (predictably called alpha, beta and gamma subunits) which are also bound to a GPCR for a total of four proteins. The alpha and gamma subunits are both membrane bound via lipids. The beta and gamma subunits always stay together (as ‘the G beta gamma subunit’). Without the extracellular ligand binding to the GPCR, the alpha subunit is bound to GDP and also bound in a complex with beta and gamma (the ‘off’ position). The ligand acts as a GEF, causing alpha to exchange GDP for GTP, whereupon it dissociates from beta and gamma and drifts away to bind to a different effector complex, causing downstream signaling
The cycle of activation and deactivation of trimeric G proteins and GPCRs is shown in this Wikimedia Commons graphic by repapetilto:
The most famous G protein coupled receptor pathways are the ones in which the effector protein that Gα binds to is adenylate cyclase (AC). AC is an enzyme which catalyzes the conversion of ATP to cAMP. Various Gα may either activate or inactivate AC, but either way, the upshot is that the GPCR acts via AC to use cAMP as a second messenger effecting downstream changes in the cell, often (always?) via binding to and activation of the Protein Kinases A (PKAs).
Here are some examples of GPCR pathways:
- Prostaglandin E1 (PGE1) is a first messenger which binds a GPCR, inhibiting AC and thus reducing cAMP, which leads to vasodilation through downstream pathways.
- Muscarinic acetylcholine receptors in heart muscle. There are several kinds of these, all of which activate a G protein. Some signal via cAMP, others open or close K+ and other channels.
- Epinephrine released in response to low blood sugar signals for an increase in intracellular cAMP, causing PKA to signal for less glycogen production and more glycogen degradation, thus freeing up stored energy in the cell in the form of glucose 1-phosphate.
- Phospholipase C (PLC) is a phosphodiesterase, which cleaves phosphatidylinositol 4,5 bisphosphate into two molecules, IP3 and DAG, which each act as second messengers in the IP3/DAG pathway. Cytosolic IP3 binds to a Ca2+ channel in the ER membrane, releasing Ca2+ stored in the ER out into the cytosol. Protein kinase C (PKC) is then activated by binding to both DAG and Ca2+, and once active it will bind and phosphorylate various substrates. Ca2+ will also cause all kinds of other downstream changes such as activating calmodulin.
Here is a video which summarizes many of the concepts of G protein signaling:
amplification of signals
Many signals are hugely amplified in the receiving cell. For instance one epinephrine molecule can activate a few ACs, each of which produces many cAMPs, each of which binds to one PKA, each of which phoshorylates many enzymes, each of which produces many end products. This sort of cascade can amplify a signal 100- to 1000-fold.
On the other hand, cells have many feedback mechanisms for downregulating or turning off incoming signals. For instance, PKAs may phosphorylate and inactivate the receptor, the cell may endocytose the receptor following a β-arrestin signal, GAPs may cause Galpha to hydrolyze ATP and return to the G protein in the ‘off’ position, or a type of phosphodiesterase (which is regulated by phosphorylation by PKA) may break down cAMP into AMP.
relevance to PrP
One of the theories as to PrP’s native function is that it’s involved in signal transduction. An oft-cited paper is Mouillet-Richard 2000 (ft), who implicated PrPC as a receptor in a signaling cascade involving Fyn (gene: FYN) and Caveolin-1 (gene: CAV1). The possible relevance of this to prion disease is not yet totally clear, but last year some fascinating new evidence came out for how this might play a role in Alzheimer’s [Larson 2012]. You’ll recall from the PrP/Aβ post that PrP has been shown to bind Aβ oligomers [Lauren 2009] and, though it’s still extremely controversial, this binding has been proposed to be necessary for Aβ toxicity, thus implicating PrP as an indispensible intermediary in Alzheimer’s pathogenesis. Meanwhile, there’s also been evidence (which Larson reviews) that Aβ activates Fyn, and that this activation is required for some aspects of Alzheimer’s pathology. Fyn is a Src tyrosine kinase, meaning that when active it phosphorylates Y residues on other proteins.
Larson’s contribution was to show that when Aβ binds PrPC, PrPC then complexes with Fyn, activating its kinase activity and causing it to phosphorylate Tau. If true, this would seem to position PrP at the long-missing causal link between the two big features of Alzheimer’s pathology: Aβ oligomers and hyperphosphorylated Tau tangles. This conclusion is sure to be controversial, and in recognition of the considerable controversy already surrounding PrP/Aβ connection, the authors were very explicit about exactly what proteins they used, obtained from where and purified how, and then what they measured and how. Such was the exhortation of a strangely anonymous opinion piece, “State of Aggregation” in Nature Neuroscience [No Authors Listed 2011] which stated that:
It is critical that studies examining the functional consequences of aggregated proteins clearly identify the exact source and aggregation state of the protein and critically discuss the implications of their approach.
Hopefully such openness will lead to some clear answers about PrP and Aβ in the near future. Larson’s evidence does look quite compelling, and may merit some further treatment on this blog after a second read.