PrP / amyloid beta interactions and the prion disease / Alzheimer's connection
 Read with caution! This post was written during early stages of trying to understand a complex scientific problem, and we didn't get everything right. The original author no longer endorses the content of this post. It is being left online for historical reasons, but read at your own risk. |
update 2013-05-07: I’ve posted an updated and more rigorous review of the PrP/Aβ literature.
Over the past decade a mixed bag of evidence has emerged regarding a possible PrP / Aβ interaction and, by extension, a possible prion disease / Alzheimer’s connection. To understand the literature on this, I first had to get up to speed on a few basics of what we know about Alzheimer’s disease.
Most cases of Alzheimer’s are late onset and sporadic. Sporadic means we don’t know why they happen, and they have no known genetic cause per se, though there are certainly risk factors, such as the APOE ε4 allele. A handful (maybe <10% or <1%) of Alzheimer’s cases are early onset and genetic, tied to mutations in either APP (amyloid precursor protein), PSEN1 or PSEN2 (the presenilins).
There’s a biological story to all this. The presenilins cleave amyloid precursor protein, resulting in N-terminal fragments 40 or 42 amino acids in length, dubbed Aβ1-40 and Aβ1-42. The disease-causing mutations in APP make it more prone to being cleaved and increase the ratio of Aβ1-42 to Aβ1-40. The disease-causing mutations in PSEN1 and PSEN2 make them cleave more APP. And apolipoprotien E, coded for by the APOE gene, is supposed to clean up Aβ fragments but the ε4 variety of the protein does a lousy job of it.
Aβ fragments form amyloid plaques in the brains of Alzheimer’s patients. The correlation between these Aβ fragments and Alzheimer’s disease is very clear, but causation is less clear. For years many people thought the plaques were the whole problem, but clinical trials of plaque-dissolving or plaque-preventing drugs (often called ‘beta breakers’ – Bay to Breakers, anyone?) have not proven successful. One school of thought is that perhaps Aβ oligomers, rather than the smaller monomers or larger plaques/aggregates, may be the more toxic species [see Benilova 2012 for a review]. On the other hand there is also new evidence that Aβ aggregates are self-propagating and transmissible – that they are, in short, prions [Stohr 2012].
Aβ is not the only peptide with a central role in Alzheimer’s. Another pathological hallmark of the disease is the formation of tau protein fibrils. Some people think tau protein is really the problem in Alzheimer’s, or that perhaps tau and Aβ both play a role. Mutations in the MAPT gene, which codes for tau protein, don’t cause Alzheimer’s, though – they cause frontotemporal dementia, which though certainly similar in a few ways is considered a totally distinct syndrome from Alzheimer’s – different brain regions are affected, different behavioral changes, different age of onset, and so on and so on.
That said, MAPT mutations are sometimes studied in mice as a model for Alzheimer’s. APP mutations do cause some tau pathology in mice in addition to Aβ plaques, but they fail to fully recapitulate the fibril formation and neuron loss. One popular Alzheimer’s model is the APPSw mice [first created by Sturchler-Pierrat 1997], which carry a human APP transgene with the Swedish double mutation (K670N + M671L, discovered by Mullan 1992) which causes early-onset Alzheimer’s. These mice have been useful for research but are an incomplete model of Alzheimer’s and so one approach has been to make double mutant mice with APP and MAPT mutations e.g. Lewis 2001.
That’s just a bit of very basic background on the proteins involved in Alzheimer’s disease. In sum, Aβ and tau are each a key part of the pathology observed in Alzheimer’s disease yet we don’t know if one, both or neither of them directly causes neuronal dysfunction and loss, or if so, by what mechanism.
One piece of evidence to suggest that Aβ directly impairs synapses comes from experiments where purified Aβ has been injected into the brains of rodents and has caused acute memory impairment. Lesne 2006 noticed that APPsw mice develop memory deficits months before their neurons start dying, suggesting Aβ can impair memory directly rather than via killing neurons. To confirm this, Lesne purified Aβ oligomers (weighing 56 kDa, so estimated to be 12-mers of Aβ1-42) from the brains of these mice and injected the oligomers into the brains of rats. Rats injected with Aβ could still traverse a maze to find a hidden platform, but couldn’t remember where the platform they had visited yesterday was. Lesne concluded that these 12-mers of Aβ impaired long-term memory recall but not spatial reasoning, and that these 12-mers were probably the toxic species of Aβ in Alzheimer’s disease (along with, perhaps, a toxic species of tau).
It was a compelling experiment, though by no means the final word – other authors also had good evidence for 2- or 3-mers [Cleary 2005]. But Lesne’s experiment provided important evidence for Aβ oligomer toxicity, and in particular, for the idea that acute memory impairment due to Aβ could be studied in rodents.
Importantly, there was already a possible mechanism for this impairment. Walsh 2002 had suggested that Aβ oligomers impair memory by suppressing long-term potentiation (LTP). Long-term potentiation is a key neuroscience concept that explains part of how we (all animals) learn. When one neuron fires repeatedly onto another one, the downstream (“post-synaptic”) neuron can become less sensitive to stimulus (habituation), or it can become more sensitive to stimulus (long-term potentiation). Both of these processes (most often studied in Aplysia) work through mechanisms involving changes in the quantity or conformation of neurotransmitter receptors and ion channels, though we don’t know all the details. Walsh delivered rapidly repeated electrical stimulus to hippocampal neurons in rats and saw that the presence of Aβ oligomers almost entirely suppressed LTP: while control neurons revved up to 150% of their original sensitivity after repeated stimulus, the neurons exposed to Aβ stayed around 100%.
A natural followup question to both Lesne and Walsh’s insights was by what mechanism Aβ suppresses LTP. Lauren 2009 observed that Aβ oligomers bind heavily to neuronal synapses – particularly to certain kinds of dendrites. To figure out what molecule Aβ was binding to on these dendrites, Lauren set up a massive assay, translating protein from 225,000 different pieces of DNA each in one well full of cells, and seeing which proteins Aβ would bind to. In that entire screen of 225,000 wells, only 2 showed binding to Aβ, and both of those were DNA coding for mouse PrP. Aβ was binding to PrP with high affinity and high specificity. Aβ did not bind to the PrP paralogs Doppel (Dpl) or Shadoo (Sprn).
To figure out what part of PrP Aβ was binding to, Lauren examined its binding to a bunch of different versions of PrP with different stretches of amino acids deleted. The best evidence suggested that Aβ was binding to PrP amino acids 95-110 (that’s THSQWNKPSKPKTNM in humans).
Since PrP appeared to be the only protein that bound with high affinity to Aβ, Lauren wondered whether PrP was a necessary part of Aβ’s memory-impairing properties. This meant repeating Walsh’s experiments in brain slices from normal mice and PrP knockout mice. In the normal control mice, repeated stimulation led to an 80% increase in synapse response measured by EPSP slope, whereas Aβ suppressed LTP, allowing only a 20% increase. In the PrP knockout mice, Aβ had no effect: neurons experienced a full ~80% increase in EPSP even Aβ was present.
In none of this research was the PrP found to be in a diseased PrPSc conformation – rather, this was ordinary PrPC carrying out what, presumably, was part of its native function. One theory Lauren mentions is the idea that PrP was facilitating Aβ to change the behavior of NMDA receptors.
But whether or not the mechanism involves NMDA, Lauren seemed to have uncovered good evidence that PrP is required for Aβ’s effects on memory.
Freir 2011 replicated Lauren’s result, again using mouse hippocampus slices to show that Aβ oligomers suppress LTP in normal but not PrP knockout mouse neurons. Freir also went a step further, using postmortem brain tissue from Alzheimer’s patients to show that Aβ oligomers suppress LTP in human neurons, and that either anti-Aβ antibodies or anti-PrP antibodies are capable of eliminating this effect and restoring LTP. What’s more, in Freir’s study, two different anti-PrP antibodies (ICSM-18 and ICSM-35) both worked – strong evidence that the LTP-restoring properties were really due to ablating PrP and not due to an off-target interaction with a different protein. (Although Barry 2011 found that antibodies against the N-terminal region of PrP could abolish Aβ LTP-impairing effects but that antibodies to the C-terminal region of PrP had no effect.)
Freir’s finding that Lauren’s results from rodents held up in human brain slices seemed a convincing confirmation of Lauren’s hypothesis. But Kessels 2010 disagreed with Lauren’s result, comparing brain slices from normal and PrP knockout mice and finding no effect of PrP on Aβ toxicity, whether in terms long term potentiation, synaptic depression or dendritic spine loss.
So after all this ex vivo work, the jury was still out. You can imagine the follow-up experiment that would be required to validate this in vivo: repeat Lesne 2006‘s experiment in PrP knockout mice.
And sure enough, a year after Laren’s paper, two groups came along and did just that experiment: Balducci 2010 and Gimbel & Nygaard 2010.
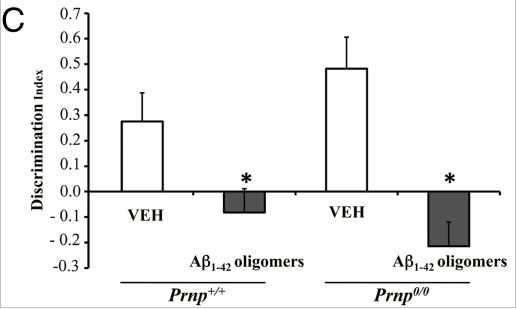
In agreement with Lauren, Balducci 2010 did find that Aβ oligomers (and again, not monomers or aggregates) bind to PrP with high affinity. And Balducci found, just as Lesne had for rats, that Aβ oligomers (but not monomers or aggregates) seemed to impair object recognition in normal mice. With regards to PrP, though, Balducci found that Aβ oligomers had the same effect on PrP knockout mice as on normal mice, as shown in Fig 4c:
Which seemed to be fairly strong evidence that PrP is not required for Aβ to be able to impair memory.
But Gimbel & Nygaard 2010 found that PrP did matter. Instead of looking at acute memory impairment from Aβ oligomer injection, though, they crossed PrP knockout mice with mice carrying two Alzheimer’s-causing transgenes: APPSw and a PSEN1 mutant, PSen1ΔE9. What they found was pretty shocking.
First, the genes acted independently in terms of protein production: levels of PrP protein didn’t depend on the presence of the two transgenes, nor vice versa. Second, PrP didn’t affect APP metabolism either: Aβ monomer production appeared to be the same in PrP wild-type and knockout mice. Third, PrP didn’t affect plaque formation: the mice with the Alzheimer’s transgenes formed the same amount of Aβ plaque whether or not they had PrP. Fourth, PrP didn’t affect astrogliosis, the brain’s inflammatory response to neurodegenerative disease – the Alzheimer’s transgenes resulted in increased numbers of astrocytes with or without PrP.
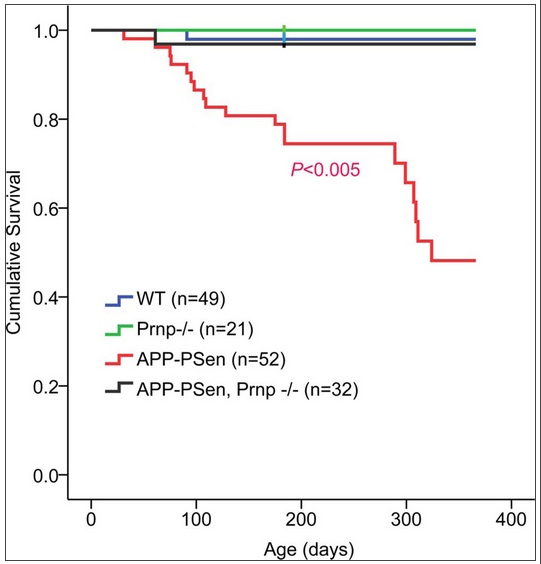
So far, it’s sounding like PrP knockout has no effect. But the Alzheimer’s mice and the Alzheimers + PrP knockout mice were different in a few very important ways. Serotonergic axons, which are usually degraded in Alzheimer’s, were spared in the PrP knockouts. Synapse loss, another hallmark of Alzheimer’s, was not seen in the PrP knockouts. Most importantly, 40% of the Alzheimer’s mice died before 12 months of age (as expected for those mice), compared to just 4% of the Alzheimer’s + PrP knockout mice – a significant difference at p = .005. The sample size is really large for a mouse study (n = 52 vs. 32) and the survival curve is striking:
Gimbel & Nygaard also did behavioral phenotyping on the mice at 12 months of age, using a water maze and a shock avoidance test. Again, the Alzheimer’s mice were impaired and the Alzheimer’s + PrP knockout mice were not.
Gimbel & Nygaard concluded that “memory deficits in AD transgenic mice require the presence of PrPC“. Their conclusion does include a lengthy discussion of how and why their findings differ from those of Balducci. Balducci’s behavioral test was based on a mouse’s choice of object to play with, relying on the fact that normal mice almost always prefer to spend time with novel objects rather than familiar ones. Balducci’s conclusions were based on the fact that Aβ-injected mice played with familiar objects, which Balducci took to mean they forgotten they’d already seen the object before. Gimbel & Nygaard argue this (1) is not really memory but rather object recognition which might be a different phenotype than memory, impaired by Aβ through a different, non-PrP-dependent pathway, and/or that (2) Balducci’s results show the mice actively preferred the familiar object, implying they hadn’t forgotten a thing, but had undergone a change of preference.
But the truth is, we don’t really know why the two studies differed. Gimbel & Nygaard’s model was a genetic one, and over the 12 months that these mice were studied, all manner of different biological and biochemical changes could have accumulated to explain the behavioral differences they observed. No one can really say if the mechanism of behavioral change in the Alzheimer’s mice involved inhibition of long-term potential like in the ex vivo experiments of Lauren 2009.
And others have failed to replicate Gimbel & Nygaard’s findings. Calella 2010 performed experiments very similar to those of Gimbel & Nygaard, with very different results. Calella crossed APPsw/Psen1ΔE9 Alzheimer’s mice with PrP knockout and with transgenic PrP overexpressers, and, in most experiments and conditions, found that neither knockout nor overexpression of PrP had any effect at all on LTP impairment in Alzheimer’s mice. The only exception to this was that in one specific condition they found that overexepression secreted PrP actually somewhat rescued neurons from the effects of Aβ – and that’s the opposite of what you’d expect based on Lauren and Gimbel & Nygaard’s studies.
So far, then, the mouse studies have been just as divided as the ex vivo studies were.
I can think of another experiment I’d like to do too: repeating Stohr 2012‘s experiment (watching prion-like propagation of Aβ in a mouse brain) in PrP knockout mice and seeing if they are resistant to Aβ prions. Based on Gimbel & Nygaard’s findings that the Alzheimer’s + PrP knockout mice did form Aβ plaques, you’d expect that Aβ transmission would be possible in PrP knockout mice but that the mice wouldn’t get behavioral symptoms of Alzheimer’s. I wager someone in the Prusiner group is doing this right now and we’ll find out the answer soon.
There have been some very specific biochemical studies trying to figure out the mechanisms by which these two proteins interact. You 2012 studied a different hippocampal system than Lauren, one where desensitization (habituation) rather than long-term potentiation was expected under normal circumstances. Excitotoxicity has been implicated as a possible cause of neurodegeneration in both Alzheimer’s and prion disease, and the failure of neurons to desensitize to repeated stimulation could be a mechanism by which neurons die. This study found that in these hippocampal neurons, any of three things: presence of Aβ, absence of copper, or absence of PrP each destroyed the neuron’s NMDA receptors‘ ability to desensitize, leading to a pathologically large continuous current that led to calcium toxicity. When Aβ was present or copper or PrP absent, the neuron, if exposed to NMDA, would just open up calcium channels until it drowned in calcium. By a lot of detailed biochemistry, the authors arrived at the conclusion that the mechanism was as follows: normally, PrP binds to copper, keeping a supply of copper ions available to inhibit the NMDA receptor’s interaction with glycine, but Aβ ruins this either by binding to PrP or by binding to copper itself.
If true, You’s theory would explain the supposedly lowered seizure threshold of PrP knockout mice [reviewed in Steele 2007] as well as the role of excitotoxicity in both Alzheimer’s and prion disease [see partial review in the memantine post], but not any of the findings that PrP knockout rescues neurons from Aβ toxicity [Lauren 2009, Freir 2011, Gimbel & Nygaard 2010].
There have been a smattering of other studies whose significance I don’t understand. Caetano 2011 found that the presence of Aβ oligomers causes a greater fraction of the cell’s PrP to cluster at the cell membrane rather than undergoing endocytosis and being anchored to membrane-bound vesicles inside the cell. Parkin 2007 found that PrP regulates the processing of APP into Aβ. Chen 2010 worked to pinpoint the Aβ binding site on PrP and the conformation of PrP needed for binding. Baier 2008 infected APPsw mice with scrapie to examine PrPSc‘s effect on production of Aβ. The most recent review I could find was Benilova 2010.
For the sake of thoroughness, I should also address the fact that it has also been suggested that PrP codon 129 MM homozygosity is associated with increased risk of Alzheimers. This correlation has been found by some authors [Riemenschneider 2004] but not others [Li 2005]. But both of those were candidate gene studies. PrP codon 129 has never once turned up as a hit in any genome-wide association studies for Alzheimer’s risk [see the NGHRI GWAS catalog (+ analysis tips here)], even though some of these studies have used enormous sample sizes (over 50,000 people in Naj 2011) and even though rs1799990, the SNP behind the 129M/V polymorphism, is always included on the SNP arrays used in these studies. For this reason I don’t believe that PrP codon 129 genotype is associated with Alzheimer’s. update: see also 2013-05-07 comment below
As for grand conclusions, we’re not quite there yet. The ex vivo studies of brain slices and in vivo studies of mice injected with Aβ or carrying Alzheimer’s mutations have roundly disagreed, reaching no consensus on whether PrP is involved in Aβ’s negative effects on learning and memory. As far as I can tell, it does appear that everyone who has studied Aβ oligomers and PrP together agrees that they bind to each other – I found no studies disputing this point. The question is whether this binding affects long-term potentiation and memory or, more broadly, whether it is relevant to the study of Alzheimer’s or prion disease in humans.
see also: May 2013 review of the PrP/Aβ literature.